Past Issues
Bioactive and Functional Groups Revealed by GC-MS and IR in Acmella Caulirhiza Modulate Inflammation and Nucleation (In Vitro Models): Implication in Kidney Stone Management
Abraham Sisein Eboh1,*, Azibanasamesa Dumaro Charles Owaba2, Ayibaene Frank-Oputu1
1Department of Biochemistry, Faculty of Basic Medical Science, Niger Delta University, Wilberforce Island Bayelsa State, Nigeria
2Department of Pharmaceutical & Medicinal Chemistry, Faculty of Pharmacy, Niger Delta University, Wilberforce Island Bayelsa State, Nigeria
*Corresponding author: Abraham Sisein Eboh, Department of Biochemistry, Faculty of Basic Medical Science, Niger Delta University, Wilberforce Island Bayelsa State, Nigeria, Phone: 08066699139, Email: [email protected]
Received Date: September 12, 2024
Publication Date: December 14, 2024
Citation: Eboh AS, et al. (2024). Bioactive and Functional Groups Revealed by GC-MS and IR in Acmella Caulirhiza Modulate Inflammation and Nucleation (In Vitro Models): Implication in Kidney Stone Management. Traditional Medicine. 5(3):30.
Copyright: Eboh AS, et al. © (2024).
ABSTRACT
Inflammation is linked to many diseases including urolithiasis or kidney stone formation. Anti-inflammatory drugs are increasing in demand due to the rising number of inflammatory linked diseases like kidney stone. Therefore, this study was undertaken to evaluate the in vitro anti-inflammatory and anti-urolithiasis ability of Acmella caulirhiza extract. The results showed a dose dependent inhibition fashion in all assays presented. Therefore, at 100 µg/ml concentration of A. caulirhiza or aspirin, the percentages inhibition of heat induced hemolysis were 97.48 ± 0.98 % and 98.91 ± 1.01 % respectively. A. caulirhiza inhibited hypotonic solution induced hemolysis and albumin denaturation. The extract inhibited trypsin at 9.65 ± 0.28 and diclofenac at 20.08 ± 1.38 % at 200 µg/ml concentration. The extract also inhibited the enzyme lipoxygenase activity and the production of thiobarbituric acid reactive substances (TBARS). The extract also inhibited nucleation in vitro at 70.32 ± 1.13 for A. caulirhiza and 87.33 ± 1.71 % for cystone at concentration of 1000 µg/ml. The phytochemicals, IR and GC-MS analysis revealed the presence of phenolics, flavonoids and alkaloids. The IR also revealed functional groups present in phenolics, flavonoids and alkaloids and other secondary metabolites and the presence of phytol, hexadecanoic acid, squalene and other phytoconstituents showed that the extract is a potential candidate for an anti – inflammatory and anti-kidney stone formation in vitro.
Keywords: Acmella caulirhiza, Inflammation, Kidney Stone, Phytochemicals, GC-MS
INTRODUCTION
Inflammation is a protection mechanism to an injury caused by microbes and other stressors. Part of this inflammatory process involves denaturation of protein, increased blood flow to the inflamed site and membrane disintegration [1]. At the end of this protection mechanism the body eliminate the stressor and return the body back to homeostasis. Inflammation entails a series of cascade marked by four cardinal signs of heat, redness, pain and swelling. The mechanism of prostaglandin synthase is central to the inflammatory process. Prostaglandin and thromboxane acts as inflammatory regulators [2]. These chemicals can lead to increased flow of blood at injury site, and recruitment of many inflammatory cells. Uncontrolled and excessive inflammation can lead to irreparable damage even though inflammation is a protective mechanism it can be more dangerous than the injury [2]. Non- steroidal anti-inflammatory drugs used against inflammatory processes possess adverse effects, irritation of gastric lining and ulcers. Hence there is the need for the search of safe, cheap, readily available and clinically effective drugs. That is why the research was sought to investigate the efficacy of Acmella cualirhiza extract in vitro. This was done using different models of anti-inflammatory processes, such as protein denaturation; heat induced hemolysis, Hypotonic induced hemolysis, trypsin inhibition, inhibition of lipoxygenase and lipid peroxidation inhibition.
Kidney stone is made of calcium oxalate (CaOx) hard materials that form in one or both of the kidneys due to the presence of certain minerals in the urine [3]. Calcium that is not metabolized by the bones and muscles goes to the kidneys and is passed out as urine, but when this process fails, kidney stones are precipitated [4]. The process of kidney stones formation is complex and is influenced by many factors such as urine concentration, pH, phosphate, certain calcium [5]. The process of kidney stones formation may begin with saturation, nucleation, crystal growth, aggregation and obstruction [6]. Obstruction can give rise to severe pain, frequent urination and blood in the urine [7]. Treatment and management of kidney stones are based on the ability of the drugs to reduce or eliminate the size of the stone. Thiazide diuretics and alkaline citrate are used frequently for treatment and management of kidney stones [8]. Other treatments include endoscopic stone removal and ureteroscopy. These procedures are very sophisticated, not common, unsafe and expensive and many cannot afford them [9]. On the other hand, medicinal plants like Acmella caulirhiza abundant in Nigeria with phytomedical properties can be an alternative.
A. caulirhiza is a flowering plant found in subtropical African countries. It is mostly found in Nigeria especially in humid soil and wasted areas. It belongs to the family Asteraceae. A. caulirhiza is called the "toothache plant" due to its tingling and numbing sensation, if flowers and buds are chewed. It has analgesic potential [10]. Most of the properties exhibited by A. caulirhiza were attributed to a chemical compound known as spilanthol an alkaloid [11]. A. caulirhiza is used for the treatment of many common ailments afflicting developing African countries such as toothache, ulcers of the mouth and sore throats [12]. Studies have also showed antibacterial activity of A. cualirhiza extract against certain pathogenic bacteria and fungi [13]. Infusions made from A. caulirhiza are used in central Kenya for the alleviation of ear, nose and throat aches [14]. The fresh leaves juice of A. cualirhiza and water is a medicine for stomach pain and diarrhea [15-16].
MATERIALS AND METHOD
A. caulirhiza preparation
The flowers of A. caulirhiza were plucked, washed and shade dried for two weeks. Later they were extracted with absolute methanol, with intermittent stiring for 72 hrs. The extract was concentrated, stored at 40C in an air tight amber colored bottle for further use.
Phytochemical screening
Qualitative phytochemical determination was carried out on the extract of A. caulirhiza to determine the presence of alkaloids, flavonoids, tannins, phenols and saponins according to the methods of [17-18].
Gas chromatography and mass spectrometry analysis
The extracted sample of A. Caulirhiza 1µl was injected into the equipment at a column temperature of 300C and heated to 3000C. The carrier gas was Helium, voltage for ionization of compound was 70eV. The mass spectrometry scan was between 45-450MHz. The unknown compounds were searched in Spectrometer data base of NIST [19].
Infrared spectroscopy analysis
The dried flower of A. Caulirhiza extract was mixed with potassium bromide and was subjected to infrared at 500 to 4000cm-1 interval.
Isolation of erythrocytes
Healthy human volunteer’s blood was drawn and centrifugrd for 10 min at 3000 rpm. The supernantant was discarded and the erythrocytes were washed thrice with equal volume of normal saline. Erythrocytes were suspended in 10% (v/v) normal saline for further use.
Heat induced hemolysis
In a 2 ml reaction mixture containing A. caulirhiza extract or Aspirin as reference (100 – 1000 µg/ml) and 1 ml of 10 % (v/v) erythrocytes in centrifuge tubes. The tubes were later warmed at 560C for 30 min. at the end of the mild heating, tubes were centrifuged at 3000 rpm for 10 min and optical density was taken at 560 nm [20].
Hypotonicity induced hemolysis
The mixture consisted of RBC (0.5 ml), 5 ml (10 mM NaCl in 10 mM phosphate buffered saline) and A. caulirhiza or the reference drug at different concentrations. The control was devoid of extract or reference. All reaction tubes were kept at room temperature for 10 min, and later centrifuged at 3000 rpm and absorbance of supernatant was read at 540 nm. Antihemolysis was calculated as percentage [21].
Albumin denaturation inhibition assay
This method was adopted from [22]. A mixture of 0.45 ml of 5 % albumin (human source), A. caulirhiza extract or diclofenac. The control tubes were devoid of extract or reference drug. The pH of the mixture was adjusted to 6.3 with 1 M HCl and later incubated at 370C for 20 min and thereafter at 570C for 3 min. Phosphate buffered saline was then added (2.5 ml) and turbidity was measured at 416 nm.
Trypsin inhibitory assay
The procedure of [23] was applied in this study. A reaction of 2000 µl contained trypsin 0.6 µg, 1 ml of A. caulirhiza extract at 100 – 1000 µg/ml and 1000 µl of tris-HCl buffer 25 mM at a pH of 7.4. The reaction was placed in a water bath at 37 0C for 5 min and casein 0.8 % w/v was added as substrate. The mixture was kept in the water bath for 20 min. later 2000 µl of of perchloric acid 70 % v/v was added to halt the enzyme activity. The mixture was centrifuged at 500 rpm for 5 min, absorbance of supernatant was read at 280 nm. Diclofenac served as standard and results were processed as percentages.
Anti-lipoxygenase activity
A. caulirhiza inhibitory activity against lipoxygenase was analyzed based on the method of [24]. A. caulirhiza and indomethacin as standard were dissolved in 2 M borate buffer solution pH 9.0 at different concentrations and 0.25 ml of lipoxygenase enzyme (2000 U/ml) were incubated for 5 min at 25 0C. later, substrate was included, agitated and absorbance was measured at 234 nm. Percentage inhibittion was calculated for A. caulirhiza and indomethacin.
Thiobarbituric acid (TBA) assay
Lipid peroxidation assay contained erythrocytes 20 % incubated with 20 mM H2O2 for 60 min at 370C in the presence of A. caulirhiza. Thereafter tubes were added 1.5 ml of 10 % TCA and centrifuged for 10 min. the supernatant was mixed with 1.5 ml of 0.67 % TBA in 50 % acetic acid. The reaction mixture was then heated for 30 min at 90 0C [25]. The developed pink color was read at 535 nm. Lipid peroxidation was expressed as percentage. Quercetin served as standard.
In Vitro Anti-kidney stones formation using nucleation assay
Anti-kidney stones formation ability of A. caulirhiza extract was analysed. Extract at different concentration was directed towards calcium oxalate (CaOx) crystallization utilizing spectrophotometry. Tris buffer 0.05M constituting CaCl2, CaOx and NaCl was warmed at 370C for 30 min. Thereafter the optical density of the tubes was measured at 620 nm [26]. The percentage anti-nucleation was then calculated.
Statistical analysis
All the data were processed and presented as mean ± S.D, using excel and GraphPad prism software.
RESULTS
Phytochemical result
|
Saponins |
++ |
|
Tannins |
+ |
|
Phenolics |
+++ |
|
Flavonoids |
++ |
|
Alkaloids |
+ |
|
Phlobatanins |
- |
key: + present; - Absent
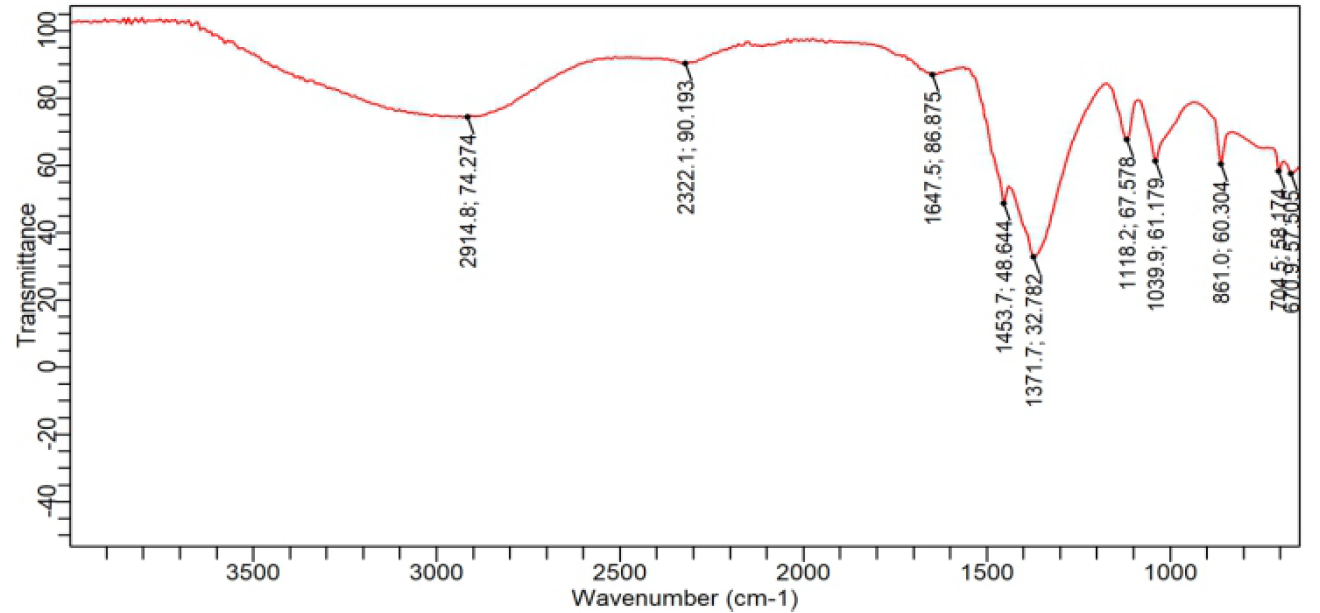
Figure 1. Showing Fourier transform infrared spectroscopy of A. caulirhiza flower extract.
The IR spectra revealed that the sample had an absorption at 2914.8 cm-1 is due C-H stretch, at 1647 is due to carbonyl group or free amide, 1453.7 cm-1, is C=C unsaturated group. While 1371 cm-1 is due to CH2 bending. These reflect that most of the chemical constituents are n-alkyl, and unsaturated derivative of organic compounds.
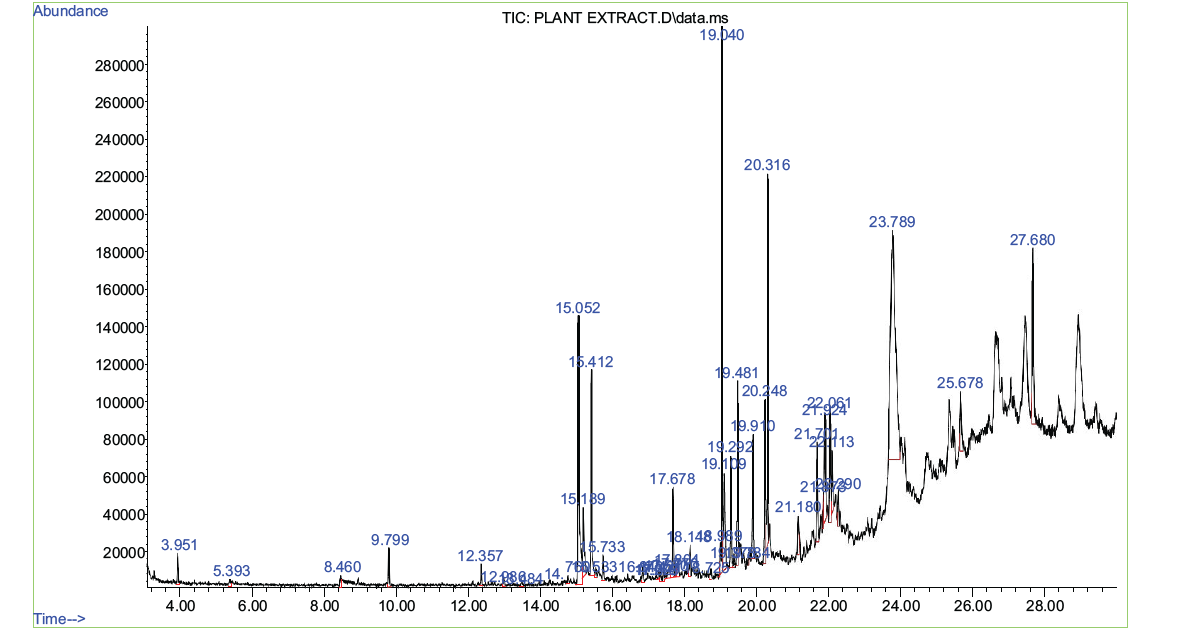
Figure 2. Showing the GC-MS chromatogram of A. caulirhiza flower extract.
Table 3. Compounds detected in GC-MS analysis of methanolic extracts of A. caulirhiza
|
Peak |
Name of compound |
Retention time |
Area% |
|
1 |
2,3-dimethyl-heptane |
3.951 |
0.61 |
|
2 |
1, 1-[(1-methyl-1, 3-propanediyl)bis(oxymethylene)]bis-Benzeneethanol, .alpha., beta. –dim ethyl- |
5.393 |
0.19 |
|
3 |
Cis-2,6-Nonadien-1-ol 3-Hexyn-1-ol |
8.460 |
0.30 |
|
4 |
2,2-Dimethyl-1-(2,4,6-trimethylphenyl)propane-1-one |
9.799 |
0.70 |
|
5 |
3-methyl-5-propyl |
12.357 |
0.34 |
|
6 |
5-methyl-Nonyl tetradecyl ether |
12.986 |
0.18 |
|
7 |
1-ethenyl-1,1,3,3-tetramethyl-3-(2-propenyl)- 4-Hydroxy-4-methyl-4H-naphthalen-1 |
13.484 |
0.17 |
|
8 |
1-bromo-Tetradecane |
14.760 |
0.19 |
|
9 |
8-epoxybenzo[7]annulene Dihydrocoumarin |
15.052 |
7.56 |
|
10 |
2-methyloctacosane |
15.189 |
1.82 |
|
11 |
2,4-Di-tert-butylphenol Phenol |
15.412 |
3.94 |
|
12 |
Tricosylpentafluoropropionate |
15.533 |
0.20 |
|
13 |
Octacosane |
15.733 |
0.53 |
|
14 |
2,6,10,14-tetramethyl-2-Bromotetradecane |
16.843 |
0.38 |
|
15 |
3,8-dimethyl-Sulfurous acid |
17.278 |
0.17 |
|
16 |
2,6,10-trimethyl- |
17.329 |
0.22 |
|
17 |
octadecyl vinyl ester |
17.421 |
0.37 |
|
18 |
Silane |
17.570 |
0.46 |
|
19 |
Heneicosane |
17.678 |
2.01 |
|
20 |
Dotriacontane |
17.770 |
0.23 |
|
21 |
Butyl dotriacontyl ether |
17.804 |
0.32 |
|
22 |
2-Bromo dodecane |
18.148 |
0.58 |
|
23 |
2,6,10-trimethyl-Oxalic acid |
18.725
|
0.19 |
|
24 |
3,7,11,15-tetramethyl-2-Hexadecene |
18.989 |
0.62 |
|
25 |
2,6,6-trimethyl-Bicyclo[3.1.1]heptane |
19.040 |
12.32 |
|
26 |
3,7,11,15-tetramethyl-2-Hexadecene |
19.109 |
2.77 |
|
27 |
3,7,11,15-Tetramethyl-2-hexadecen- 1-ol 1,4-Eicosadiene |
19.292 |
2.34 |
|
28 |
5-ethyl-1,3-dioxan-5-yl isobutyl ester
|
19.378 |
0.25 |
|
29 |
1-ethynylcyclohexanol |
19.481 |
3.94 |
|
30 |
2,4,6-trimethyl Decane |
19.784 |
0.23 |
|
31 |
Tetracosane |
19.910 |
2.74 |
|
32 |
n-Hexadecanoic acid |
20.248 |
4.10 |
|
33 |
Dibutyl phthalate |
20.316 |
7.28 |
|
34 |
(1S,2R,4R,7R)-4-Isopropyl-7-methyl-3,8-dioxatricyclo[5.1.0.02,4]octane
|
21.180 |
0.77 |
|
35 |
Phytol |
21.701 |
2.19 |
|
36 |
Methoxyacetic acid |
21.873 |
0.50 |
|
37 |
Hentriacontane |
21.924 |
2.86 |
|
38 |
Dimethyl(dimethylpentyloxysilyloxy)hexyloxy-Silane |
22.061 |
2.45 |
|
39 |
Octadecanoic acid |
22.113 |
1.34 |
|
40 |
Tetrapentacontane |
22.290 |
0.85 |
|
41 |
1,1'-(2-propyl-1,3-propanediyl)bis-cyclohexane |
23.789 |
23.82 |
|
42 |
Benzo[h]quinoline-2,4-dimethyl |
25.678 |
1.82 |
|
43 |
Squalene |
27.680 |
5.17 |
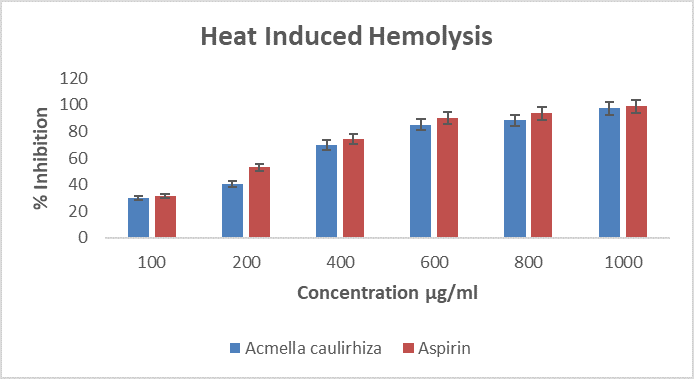
Figure 3. Showing heat induced hemolysis of erthrocytes, in the presence of A. caulirhiza and the standard drug (aspirin) at different concentrations. The pattern of inhibition was concentration dependent. At dose of 100 µg/ml A. caulirhiza inhibited 29.93 % while the standard drug inhibited 31.43 %. There was significant difference between the plant extract and the standard drug (P<0.05).
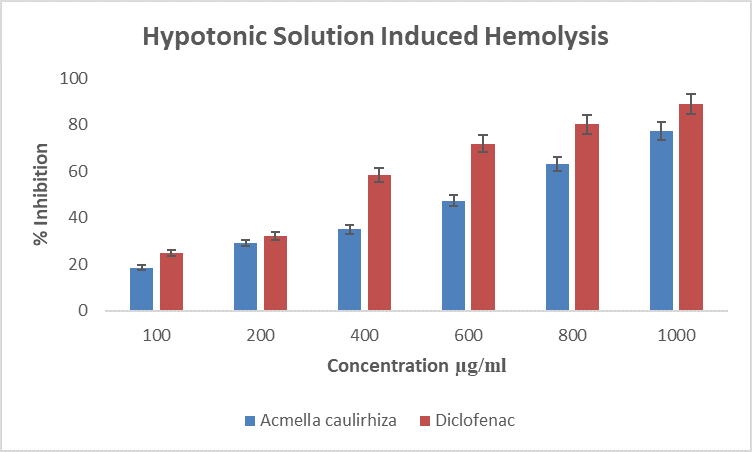
Figure 4. Showing hypotonic solution induced hemolysis of erthrocytes, in the presence of A. caulirhiza or the standard drug (diclofenac) at different. The pattern of inhibition was concentration dependent. At a dose of 100 µg/ml A. caulirhiza inhibited 18.61 % while the standard drug inhibited 25.01 %. There was significant difference between the plant extract and the standard drug (P<0.05).
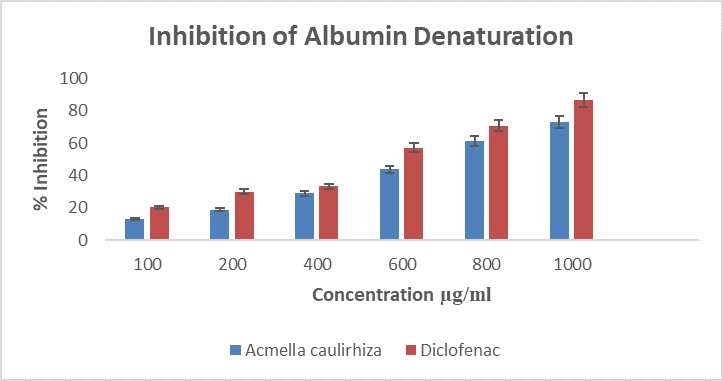
Figure 5. Showing inhibition of albumin denaturation, in the presence of A. caulirhiza and the standard drug (diclofenac) at different concentrations. The pattern of inhibition was concentration dependent. At a dose of 100 µg/ml A. caulirhiza inhibited 13.21 % while the standard drug inhibited 20.16 %. There was significant difference between the plant extract and the standard drug (P<0.05).
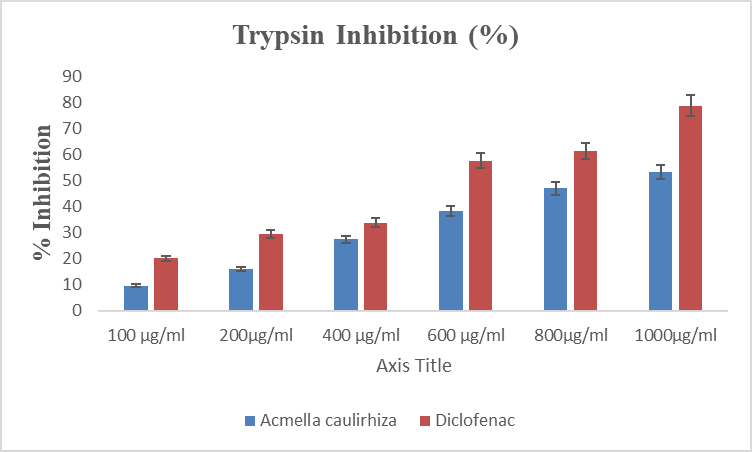 Figure 6. Showing % inhibition of trypsin, in the presence of A. caulirhiza and the standard drug (diclofenac) at different concentrations. The pattern of inhibition was concentration dependent. At a dose of 100 µg/ml A. caulirhiza inhibited 9.65 % while the standard drug inhibited 20.08 %. There was significant difference between the plant extract and the standard drug (P<0.05).
Figure 6. Showing % inhibition of trypsin, in the presence of A. caulirhiza and the standard drug (diclofenac) at different concentrations. The pattern of inhibition was concentration dependent. At a dose of 100 µg/ml A. caulirhiza inhibited 9.65 % while the standard drug inhibited 20.08 %. There was significant difference between the plant extract and the standard drug (P<0.05).
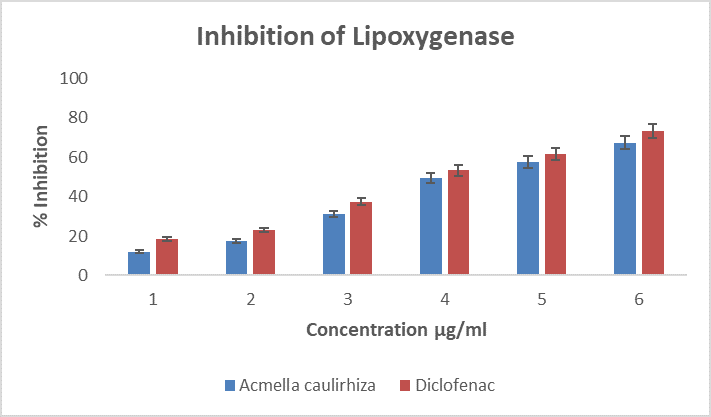
Figure 7. Showing % inhibition of lipoxygenase, in the presence of A. caulirhiza and the standard drug (diclofenac) at different concentrations. The pattern of inhibition was concentration dependent. At a dose of 100 µg/ml A. caulirhiza inhibited 12.11 % while the standard drug inhibited 18.63 %. There was significant difference between the plant extract and the standard drug (P<0.05).
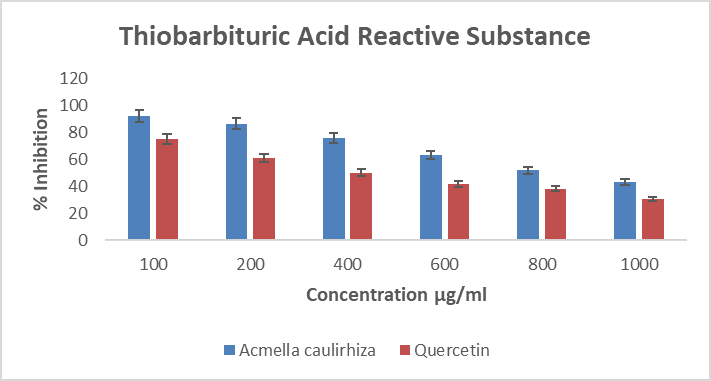
Figure 8. Showing % inhibition of thiobarbituric acid reactive substance, in the presence of A. caulirhiza or the standard drug (quercetin) at different concentrations. The pattern of inhibition was concentration dependent. At a dose of 100 µg/ml A. caulirhiza inhibited 91.87 % while the standard drug inhibited 74.71 %. There was significant difference between the plant extract and the standard drug (P<0.05).
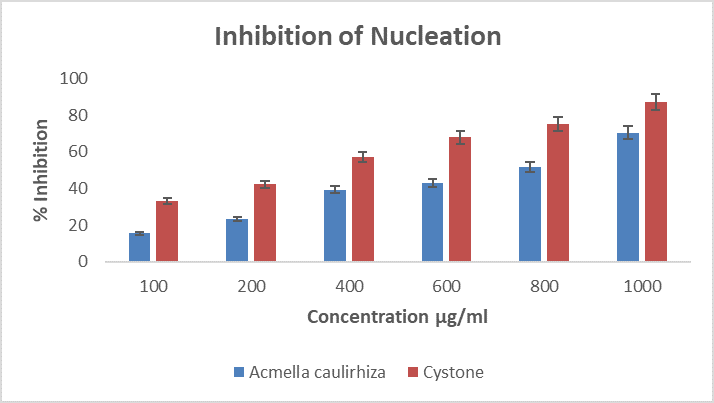
Figure 9. Showing % inhibition of nucleation, in the presence of A. caulirhiza and the standard drug (cystone) at different concentrations. The pattern of inhibition was concentration dependent. At a dose of 100 µg/ml A. caulirhiza inhibited 15.63 % while the standard drug inhibited 33.22 %. There was significant difference between the plant extract and the standard drug (P<0.05).
DISCUSSION
Qualitative phytochemical determination was carried out to detect the presence of secondary metabolites. In the present study, A. caulirhiza extract revealed the presence of phenolics, flavonoids and alkaloids. The IR revealed functional groups like C=C, C-H, CO. CNH and CH2 these groups revealed the presence of phenols, flavonoids and alkaloids that are found in the extract of A. caulirhiza. These results are similar to the findings of [27], who also reported functional groups in the extract of Capparis divaricate. The GC-MS analysis of extract revealed a lot of abundant biocompounds which are 43 in number but those that are more abundant are squalene, benzo[h]quinolone-2,4-dimethyl, octadecanoic acid, phytol, hexadecanoic acid, 2,4-ditertbutyl phenol, 8-epoxybenzo [7] annulene dihydrocoumarin, dibutyl phthalate etc. these compounds have been reported to have antioxidant, anticancer, anti-inflamatory, antibacterial and antidiabetic properties [28-31].
Protein denaturation involves disruption of forces that stabilizes secondary, tertiary and quaternary levels of protein arrangement which could lead to the death of cells. Denaturing agents include heat, salt, acidity, stress etc. denaturation of proteins lead to inflammation and in turn to cancer, ulcer, cataract etc. [32]. To investigate the mechanism underlying the ability of A. caulirhiza to act as anti-inflammatory drug, the extract was subjected to protein denaturation inhibition assay in vitro. The result showed that extract has potentials to inhibit denaturation as showed in figure 5 above; this ability of the extract could be due to the phytochemicals present in the extract of A. caulirhiza that can regulate the activities of auto-antigens [33]. These results are also like the reports of [34] who also reported anti-albumin denaturation by the extract of Murraya koenigii.
Protection of the membrane of lysosomes is key to anti-inflammatory processes, because lysis of the lysosomal membrane which resembles erythrocyte membrane leads to inflammation [35]. Hypotonicity and heat induced hemolysis may be by squeezing the cell membrane due to osmotic discharge of cellular electrolyte and fluid [36]. The ability of extracts to inhibit this process in erythrocytes is by either preventing damage to the membrane or efflux of intracellular components [37]. The components in these extracts are responsible for this inhibitory activity, which are phytochemicals. Many active compounds were found in A. caulirhiza which are also responsible for the maintenance of erythrocyte membrane integrity when induced by heat or hypotonic solution. Our reports are similar to the reports of [35].
Protease inhibitors are useful drugs for the treatment of many inflammatory related diseases like rheumatoid arthritis, cancer, AIDS, thrombosis and pancreatitis [38]. Proteases are rich in lysosomes of neutrophils. They play cardinal role in the development of tissue damage during the inflammatory process [39]. In inflammatory processes trypsin is also activated in the initial process [40], in the present study trypsin is inhibited by A. caulirhiza in a concentration dependent fashion. Our reports are similar to the findings of [35,33].
Nucleation is the rate limiting step in the process of kidney stone formation, nucleation begins when there is a combination of stone salt in the urine with other new stones like calcium chloride and calcium oxalate, this then result in calcium oxalate formation [41]. Saponin abundant fraction of the plant extract Herniaria hirsuta was reported to inhibit nucleation in vitro [42]. The present phytochemical result of A. caulirhiza showed the presence of saponins and the anti-nucleation result revealed that A. caulirhiza inhibited calcium oxalate nucleation in vitro. These reports are similar to the work of [43].
REFERENCES
- Sadique J, Chandra T, Thenmozhi V and Elango V. (1987). The anti-inflammatory activity Enicostemma littorale and Mullogo cerviana. Biochem Med Metab Biol. 37(2):167-176.
- Gerard JT, Sandra R. (1993). Principles of Anatomy and Physiology. Harper Collins College Publishers, 7th edition. pp 695.
- Khan SR, Pearle MS and Robertson WG. (2016). Kidney stoney. Nat Rev D.S. primers. 25(2):16008.
- Ferraro PM, Curhan GC, D'Addessi A, Gambaro G. (2017). Risk of recurrence of idiopathic calcium kidney stones: analysis of data from the literature. J Nephrol. 30(2):227-233.
- Johnson M, White L, Thompson G. (2019). Vitamins and their role in kidney stone prevention. Nutrition and health. 23(3):278-290.
- Johnson M, White LG. (2019). Vitamins and their role in kidney stone prevention. Nutrition and health. 23(3):278-290.
- Pearle MS, Calhoun EA, Curhan GC; Urologic Diseases of America Project. (2005). Urologic diseases in America project: urolithiasis. J Urol. 173(3):848-857.
- Galuni VJ, Panchal RR. (2014). In vitro evaluation of centratherum anthelminticum seed for antinephrolithiatic activity. Journal of Homeopathy and Ayurvedic medicine. 3(1).
- Pise MV, Sarkar S, Dubey R, Paclye R. (2018). Synadenium grantii leaf extract decrease the aggregation, nucleation and formation of urinary Crystals. European journal of biomedical and pharmaceutical science. 5(8):435-440.
- Silva J, Abe S, Murakami T. (2016). Acmella caulirhiza extract: An alternative to decrease the pain threshold of the with pilpitis. Phytotherapy Research. 30(12):2029-2033.
- Santos-filho PR. (2017). Spilanthol: occurrence, extraction, chemistry and biological activities. Revista Brasileira de Farmacognosia. 27(1):109-122.
- Chattopadhyay D. (2015). Ethnopharmacological survey of traditional medicinal plants in the Eastern Himalaya a region of Arunachal Pradesh, India. J Ethnopharmacol. 171:86-89.
- Prachayasittikul S, Prachayasittikul V, Ruchirawat S. (2013). High therapeutic potential of Spilanthes acmella: A review. Excli Journal. 12:291-312.
- Njoroge GN, Bussmann RW. (2006). Traditional management of ear, nose and throat (ENT) diseases in central Kenya. J Ethnobiol Ethnomed. 2(1):1-9.
- Chhabra SC, Mahunnah RLA, Mshiu EN. (1989). Plants used in traditional medicine in Eastern Tanzania. Angiosperms (Capparidaceae to Ebenaceue). J Ethnopharmacol. 25(3):339-359.
- Giday M, Astaw Z, Woldu Z. (2010). Ethnomedicinal study of plants used by Sheko ethnic groups of Ethiopia. J Ethnopharmacol. 132(1):75-85.
- Evans WC. (2009). Trease and evans pharmacognosy. International Edition E-Book 614 pages. 6th edition. Elsevier Health Sciences, London, UK.
- Sofowora A. (1993).‘ Phytochemical Screening of Medicinal Plants and Traditional Medicine in Africa, Spectrum Books Ltd., Ibadan, Nigeria.
- Kumar D, Rajakumar R. (2016). Gas chromatography, mass chromatography analysis of bioactive component from the Ethanol extract of Avicennia marina leaves. Innovare J Sci. 4(4):9-12.
- Sadique J, Al-Rqobahs WA, Bughaith, EIGindi AR. (1989). The bioactivity of certain medicinal plants on the stabilization of RBS membrane system. Fitoterapia. 60:525-532.
- Seeman P, Weinstein J. (1966). Erythrocyte membrane stabilization by tranquilizers and antihistamines. Biochem Pharmacol. 15(11):1737-1752.
- Mizushima Y, Kobayashi M. (1968). Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharma Pharmacol. 20(3):169-173.
- Oyedapo O, Famurewa A. (1995). Antiprotease and membrane stabilizing activities of extracts of Fagara zanthoxyloides, Olax subscorpioides and Tetrapleura tetraptera. International Journal of Pharmacognosy. 33(1):65-69.
- Shinde UA, Kulkarni KR, Phadke AS, Nair AM, Dikshit VJM, Saraf MN. (1999). Mast cell stabilizing and lipoxygenase inhibitory activity of Cedrus deodara (Roxb,) Leud. Wood oil. Indian J Exp Biol. 37(3):258-261.
- Mendanha SA, Anjos JLV, Silva AHM, Alonso A. (2012). Electron paramagnetic resonance study of lipid and protein membrane components of erythrocytes oxidized with hydrogen peroxide. Braz J Med Biol Res. 45(6):473-481.
- Bawari S, Negi-Sah A, Tewari D. (2018). Antiurolithiatic activity of Daucus carota: an in vitro study. Pharmacogn J. 10(5):880-884.
- Lalitha P, Parthiban A, Sachithanandam V, Purvaja R, Ramesh R. (2021). Antibacterial and antioxidant potential of GC-MS analysis of crude ethyl acetate extract from the tropical mangrove plant Avicennia officinalis L. South African Journal of Botany. 142:149-155.
- Mohammad SN, Srinivasulu A, Chittibabu B, Rao UMV. (2015). Isolation and purification of antibacterial principle from Avicennia marina L in methanol. Int J Pharm Pharm Sci. 7(1):38-41.
- Kaliamurthi S, Selvaraj G. (2016). Insight on Excoecaria agallocha: An Overview. Nat Prod Chem Res. 4(2):1000203.
- Joel EL, Bhimba V. (2010). Isolation and characterization of secondary metabolites from the mangrove plant Rhizophora mucronata. Asian Pacific J Trop Med. 3(8):602-604.
- Sobolev VS, Khan SI, Tabanca N, Wedge DE, Manly SP, Cutler SJ, et al. (2011). Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic Stilbenoids. J Agric Food Chem. 59(5):1673-1682.
- Vallabh D, Varsha JM, Kadam VJ. (2009). In vitro anti-arthritic activity of Abitilam indicu (linn) sweet. J Pharm Re. 2(4):644-645.
- Saleem A, Saleem M, Akhtar MF. (2020). Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. South African Journal of Botany. 128:246-256.
- Perera HDSM, Samarasekera JKRR, Handunnetti SM, Weerasena OVDSJ. (2016). In vitro anti-inflammatory and antioxidant activities of Sri Lankan Medicinal plants. Industrial Crops and Products. 94:610-620.
- Shilpa K, Nimmy C, Prerana S, Sandhya SA. (2018). Investigation of anti-arthritic activity (in vitro models) of Hibiscus hispidissimus Griffith. The Journal of Phytopharnacology. 7(1):60-65.
- Jothi BM, Yagananth N. (2011). In vitro anti-inflammatory activity and tissue culture studies on Andrographis paninculata (Burm. F.) Wallich Ex. Nees; A medicinal plant. Journal of Pharmacy Research. 4(5):1368-1369.
- Oyeleke SA, Ajayi AM, Umukoro S, Aderibigbe A, Ademowo OG. (2018). Anti-inflammatory activity of theobroma cacoa stem bark ethanol extract and its fractions in experimental models. J Ethnopharmacol. 10(222):239-248.
- Saldanha E, Pai RJ, George T, D’Souza S, Adnan M, Pais M, et al. (2018). Health effects of various dietary agents and phytochemicals (therapy of acute pancreatitis). Therapeutic Probiotics and Unconventional Foods. Elsevier. pp. 303-314.
- Das SN, Chatterjee S. (1995). Long term toxicity study of ART-400. Indian Indg Med. 16(2):117-123.
- Pemiah B, Reshma AKP. (2014). In-vitro anti-inflammatory, antioxidant and nephroprotective studies on leaves of Aegle marmelos and Ocimum sanctum. Asian J Pharm Clin Res. 7(4):121-129.
- Aggarwal KP, Narula S, Kakkar M, Tandon C. (2013). Nephrolithiasis: molecular mechanism of Renal stone formation and a critical role played by modulators. Biomed Res Int. 213(21):123-131.
- Fouada A, Yamina S, Nait MA, Mohammed B, Abdlekrim R. (2006). In vitro and in vivo antilithiatic effect of saponin rich fraction isolated from Herniaria hirsuta. J Bras Nefrol. 28:199-203.
- Chaudhary D, Paudel S, Rana RM, Timsina S, Malla KP, Giri PM, et al. (2018). Inhibition of calcium oxalate crystallization in vitro by methanolic leaf extract of Murraya koenigii (L.) Spreng. Int J Herb Med. 6(2):13-15.
 Abstract
Abstract  PDF
PDF