Past Issues
Medicinal Plants Used for the Treatment of Common Parasitic Diseases by Traditional Practitioners in Kabul City, Afghanistan
Rabia Ayoubi1,*, Amir Kabir Raufi2, Bashir Ahmad Bashir3, Hadiqa Nowrozi4
1Department of Pharmacognosy, Faculty of Pharmacy, Kabul University, Afghanistan
2 Department of Pharmacy, Ghazanfar Institute of Health Sciences, Afghanistan
3Department of Biochemistry-Nutrition, Faculty of Pharmacy, Kabul University, Afghanistan
4Pharm- D, Faculty of Pharmacy, Kabul University, Afghanistan
*Corresponding author: Rabia Ayoubi, Department of Pharmacognosy, Faculty of Pharmacy, Kabul University, Afghanistan, Tel: +93790105386; E-mail: [email protected]
Received Date: May 10, 2024
Publication Date: June 06, 2024
Citation: Ayoubi R, et al. (2024). Medicinal Plants Used for the Treatment of Common Parasitic Diseases by Traditional Practitioners in Kabul City, Afghanistan. Traditional Medicine. 5(1):22.
Copyright: Ayoubi R, et al. © (2024).
ABSTRACT
The use of traditional medicine (TM) methods, especially phytotherapy, for the treatment of most common and dangerous infectious diseases such as parasitic diseases (PDs) is common in many developing countries worldwide, including Afghanistan. During many centuries, humans have suffered many injuries from parasites, and PDs, such as yellow fever, malaria and hookworm infections in tropical, poor and developing countries which are among the main causes of human mortality. The main purpose of this research was to introduce medicinal plants (MPs) used for the treatment of common PDs by traditional practitioners (TPs) in Kabul city. The study was a field research conducted on the months of August and September 2020. Kabul city was selected as the field of study. Cross-sectional convenience sampling was used for data collection. The required information was randomly collected from seven districts of Kabul city and accepted as sample. TPs were selected as participants and interviewed using prepared questionnaires. The results of this study showed that a total of 29 MPs from 16 plant families were used by TPs for the treatment of common PDs, and mostly the underground parts, herbs, fruits and leaves of the mentioned plants were used in the form of decoction, infusion, extracts and powdered for this purpose. The effectiveness of using MPs to treat PDs by TPs in Kabul city, in most of the cases, matches the rational phytotherapy. However, further studies are needed to ensure the identity, purity, quality and uniformity of MPs and their recommended dosage forms throughout the city.
Keywords: Parasitic Diseases, Medicinal Plants, Traditional Medicine, Kabul city, Afghanistan.
INTRODUCTION
The use of medicinal plants (MPs) for the treatment of various diseases is consistent with human history. Since ancient times, MPs have been the only accessible choice humans used for the treatment and management of diseases and for many years, natural remedies, especially herbal medicines, have been used as the basis and even in some cases the only available method of this purpose [1].
For a long time, traditional medicine (TM) has been the usual method of self-treatment of people and it is easily acceptable for them. TM emphasizes the fact that low-cost herbal medicine, under the supervision of professionals, does not have side effects and is considered one of the best treatment strategies [2]. Preparation and processing of MPs are easier compared to the chemical alternatives and their maintenance is also simple. In addition, these drugs strengthen the body's immune system. Since the application of TM approaches can be influenced by cultural and historical factors and the individual and philosophical views of the people, so the above-mentioned methods have shown variation in different countries.
The usage of synthetic and chemical medicines for the prevention, control and treatment of various diseases commonly causes several side effects and in some cases adversely affect the body's immune system. While, many of the methods used in TM contains experiences that are inherited and demonstrated their safety and efficiency in a beneficial way.
The use of TM methods, especially phytotherapy, for the treatment of most common and dangerous infectious diseases such as parasitic diseases (PDs) is usual in many developing countries of the world, including Afghanistan.
A parasite is a living organism that spends its life completely or partially on another living organism and obtains the food it needs from it. Parasites are protozoa, worms and some insects. The three main classes of parasites that cause diseases in humans include protozoa, worms, and ectoparasites. During many centuries, humans have suffered much harm from parasites. Insects such as cockroaches and their symbiotic bacteria caused the mortality of one third of the European population in the 17th century [3]. Malaria, schistosomiasis and African trypanosomiasis have killed millions of people. Even today, despite extensive campaigns and remarkable progress against diseases such as yellow fever, malaria, and hookworm infections in many parts of the world, PDs in tropical, poor and developing countries remains as the main causes of human mortality [4]. In 1997, the global prevalence of diseases caused by the parasites was recorded as follow: Ascaris Lumbricoides 24%, Trichuris Trichiura 17% and hookworms 24%. These figures have remained objectively stable for fifty years despite the increase in the world population [3].
The increase in the possibility of the spread of PDs, especially in poor and densely populated areas, the high level of mortality caused by these diseases, the lack of availability and accessibility of safe and effective anti-parasitic drugs, the high cost and sometimes unbearable side effects of the treatment regimens for PDs , the emergence of parasitic resistances against chemical anti-parasitic medicines, etc. are among some of the reasons that have led the majority of people to still rely on and accept phytotherapy and TM for the treatment of PDs, despite the considerable developments that has happened in the field of synthetic alternatives [5].
TM is valued highly in Afghanistan as well as in other countries. The people of the country still use MPs despite the rapid growth of synthetic drugs. The rich plant flora and the history and fame of TM in Afghanistan have made the majority of the people to visit traditional practitioners (TPs) for the treatment of their mental and physical diseases and use herbal remedies.
Considering the national and global importance of fight against the spread of PDs, the urgency of the prevention, control and treatment of PDs by focusing on the popular methods of treating these diseases by TPs in the country, a decision was made to investigate the use of MPs for the treatment of PDs in different districts of Kabul city, one of the most populated provinces of the country. The main purpose of this research was to introduce MPs used in the treatment of common PDs by TPs in different districts of Kabul city, Afghanistan.
Aims and Objectives
This research aimed to investigate the traditional phytotherapy for the treatment of common PDs employed by TPs across the different districts of Kabul city. In most specific words, the main objectives of the study were:
- To introduce MPs and their recommended preparations used for the treatment of common PDs by TPs in different districts of Kabul city, Afghanistan.
- To compare the effectiveness of MPs used for the treatment of common PDs with the evidence-based phytotherapy.
Method
The study was a field research conducted on the months of August and September 2020. Kabul city was selected as the study area. Cross-sectional convenience sampling was used to collect data. The required information was randomly collected from seven districts (the first, second, fourth, fifth, sixth, thirteenth, and eighteenth districts) of Kabul city and was accepted as sample. TPs were selected as participants and interviewed using pre-prepared questionnaires. The prepared questionnaires included the information regarding the demographic characteristics of TPs, the type of MPs used for the treatment of PDs by TPs, the MPs parts used for the treatment of PDs and their methods of preparations, the reasons for using herbal medicine for the treatment of PDs and some other general questions.
Results
The results of this research showed that a total of 29 MPs were used by TPs in the first, second, fourth, fifth, sixth, thirteenth and eighteenth districts of Kabul city, Afghanistan for the treatment of common PDs including amoebiasis, leishmaniasis, malaria, ascariasis and other worm diseases. The used MPs along with their scientific and local names, plant families, parts used and method of preparations are summarized in Table 1.
Table 1. List of MPs used for the treatment of PDs by TPs in Kabul city, Afghanistan
|
No |
Scientific Name |
Local Name |
Plant Family |
Part Used |
Method of Preparation |
|
1 |
Zingiber officinale Roscoe |
Zangabil |
Zingiberaceae |
Rhizome |
Infusion, Decoction |
|
2 |
Curcuma longa L. |
Zardchoba |
Zingiberaceae |
Rhizome |
Decoction |
|
3 |
Achillea sp. |
Boy madaran |
Asteraceae |
Dried herb |
Tea |
|
4 |
Artemisia cina berg |
Terkh, Kerm buta |
Asteraceae |
Flower buds |
Powder, Infusion |
|
5 |
Cichorium intybus L. |
Kasni |
Asteraceae |
Root |
Decoction |
|
6 |
Artemisia absenthium L. |
Afsentine, Marwa |
Asteraceae |
Leaves and herb |
Decoction, Infusion |
|
7 |
Ferula assa-foetida L. |
Hing, Anghoza, Anjawan |
Apiaceae |
Latex |
Latex+ Yogurt |
|
8 |
Carum copticum L. |
Jawani, Spirkay |
Apiaceae |
Fruits |
Decoction, Infusion, Powder |
|
9 |
Foeniculum vulgare Mill. |
Badyan |
Apiaceae |
Fruits |
Decoction, Infusion |
|
10 |
Coriandrum sativum L. |
Gashniz, Gashnich |
Apiaceae |
Fruits |
Infusion, Tea |
|
11 |
Anethum graveolens L. |
Shebet, Shewet |
Apiaceae |
Fruits and herb |
Decoction, Extract |
|
12 |
Caryophyllus aromaticus L. |
Mekhak |
Myrtaceae |
Unbloomed flowers |
Decoction |
|
13 |
Eucalyptus sp.. |
Eucalyptus |
Myrtaceae |
Leaves |
Tea |
|
14 |
Glycyrrhiza glabra L. |
Shirin boya, Shirin bayan |
Fabaceae |
Underground parts |
Powder |
|
15 |
Tamarindus indica L. |
Tamr-e-hindi |
Fabaceae |
Fruits |
Tea |
|
16 |
Cucurbita pepo L. |
Kadoo |
Cucurbitaceae |
Seeds |
Powder |
|
17 |
Nigella sativa L. |
Sia dana |
Ranunculaceae |
Seeds |
Dried fruits |
|
18 |
Punica granatum L. |
Anar |
Lythraceae |
Bark, Root, Stem and Twigs |
Infusion, Extract |
|
19 |
Linum usitatissimum L. |
Zegher, Katan |
Linaceae |
Seeds |
Decoction |
|
20 |
Fumaria sp. |
Shahtara |
Papaveraceae |
Herb |
Extract |
|
21 |
Cinnamomum zeylanicum Blume |
Dalchini, Darchini, Siloni |
Lauraceae |
Dried stem/s bark |
Infusion, Extract |
|
22 |
Cassia angustifolia M. Vahl |
Senna |
Fabaceae |
Leaves and Fruits |
Decoction, Infusion |
|
23 |
Trigonella foenum-graecum L. |
Shanbalila |
Fabaceae |
Seeds |
Dried seeds |
|
24 |
Aloe sp. |
Sabr-e-zard |
Liliaceae |
Leaves |
Gel |
|
25 |
Plantago major L. |
Zoof, Espaghol |
Plantaginaceae |
Dried leaves and Fresh herb |
Powder |
|
26 |
Lavadula sp. |
Ostokhodos, Lavanda |
Lamiaceae |
Fresh inflorescence |
Decoction |
|
27 |
Chelidonium majus L. |
Mamiran, Mamirak |
Papaveraceae |
Herb |
Latex |
|
28 |
Bistorta officinalis Delarbre |
Anjabar |
Polygonaceae |
Leaves, Rhizome and Root |
Extract |
|
29 |
Embelia ribes Burm. f. |
Barang-e-kabuli, Birang, Barnaq |
Primulaceae |
Fruits |
Dried fruits |
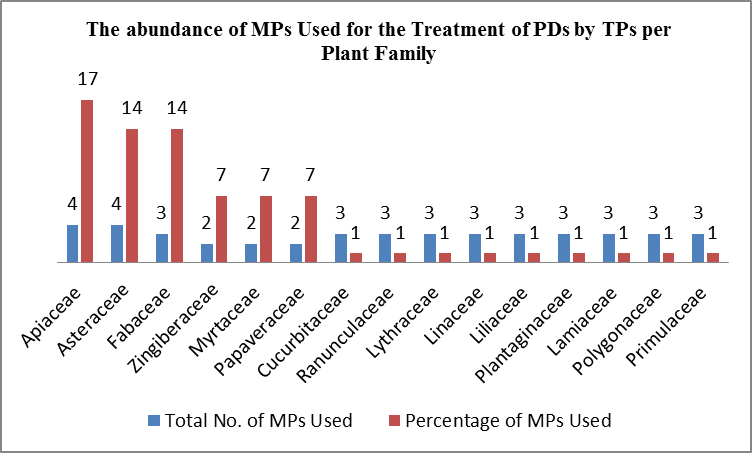
Figure 1. The abundance of MPs used for the treatment of PDs by TPs in different districts of Kabul city per plant family (researcher).
As mentioned in Table 1, totally 29 MPs belonging to 16 plant families have been used by TPs in different districts of Kabul city to treat common PDs. Of these, most of these MPs (them) belonged to the plant families Apiaceae (17%), Asteraceae (14%) and Fabaceae (14%), and the rest belonged to other plant families (Figure 1).
The most common method of preparations recommended for the treatment of PDs by the TPs of Kabul city were decoction (30%), infusion (24%), extract (14%) and dry powders (14%) and other less commonly used forms included tea, the combined forms of drugs (hing + yogurt), dry fruits and seeds and gel (19%). TPs have recommended decoction forms of MPs orally, in most of the cases. The dosage and the administration frequency of different preparations were decided based on the severity of the disease, the overall health condition of the patient, the occurrence of any kind of side effects and allergic reactions etc. TPs of Kabul city have used different parts of the MPs, including underground parts (19%), herbs (19%) and fruits (19%), leaves (17%), seeds, flowers/flower buds, bark and resin (26%) to treat PDs.
DISCUSSION
There are a number of MPs used by TPs for the treatment of various diseases; including PDs. MPs attract much attention due to their complex composition, high effectiveness, availability, lesser side effects and low price.
PDs are diseases that are caused by parasites and create various complications and even death. Considering the results of this research, which was conducted in order to introduce and study the MPs used for the treatment of common PDs by TPs in the first, second, fourth, fifth, sixth, thirteenth and eighteenth districts of Kabul city, it was revealed that a total of 29 MPs including Z. officinale, C. longa, Achillea sp., A. cina berg, C. intybus, A. absenthium, F. assa-foetida, C. copticum, F. vulgare, C. sativum, A. graveolens, C. aromaticus, Eucalyptus sp., G. glabra, T. indica, C. pepo, N. sativa, P. granatum, L. usitatissimum, Fumaria sp., C. zeylanicum, C. angustifolia, T. foenum-graecum, Aloe sp., P. major, Lavadula sp.., C. majus B. officinalis and E. ribes are used to treat common PDs such as ascariasis, amoebiasis, malaria, leishmaniasis and diseases caused by roundworms in the selected areas. This is while the anti-parasitic effects of most of the aforementioned plants have been already proven by separate scientific research.
TPs have used different types of preparations, including decoction of MPs (orally in most of the cases) for the treatment purposes. The dosage and the administration frequency of different types of preparations adjusted according to the severity of the disease, the overall health condition of the patient, the occurrence of any kind of side effects and allergic reactions etc.
The results of this study showed that TPs mostly use the powdered, infusion and decoction of Z. officinale to treat amoebiasis. Other studies have shown the anthelmintic, effects against the worms Shistosoma mansoni, Hymenolepis nana and Toxocara canis [6-8]; anti-protozoa, activities against Giardia lambelia, Trypanosoma brucei and Toxoplasma gondii [9,10]; and in-vitro effectiveness of this plant against cystic echinococcosis [11]. The essential oil of Z. officinale is responsible for its anti-parasitic effects.
TPs in different districts of Kabul city have used C. longa decoction to treat PDs. Meanwhile, the extract of this plant along with Aloe sp. has been used for the treatment of chronic leishmaniasis [12] and its ethanolic extract has good efficacy against Plasmodium vivax resistant to chloroquine [13]. Curcumin one of the main phytoconstituents of C. longa prevents the enzymatic activity of Tg Glo1 and has been effective in the treatment of toxoplasmosis [14].
The dried herb of Achillea sp. in the form of tea or infusion has used by the TPs of Kabul city for the treatment of diseases caused by roundworms and also malaria. The results of the researches conducted on A. millefolium have shown that the essential oil of this plant is used to eliminate the causative agents of malaria, Chagas, toxoplasmosis and hydatid diseases and the chamazulene present in the essential oil of the plant is responsible for its anti-parasitic effects [15]. In addition, the A. millefolium has anti-malarial, anti-hepatitis and anti-jaundice effects [16]. Based on the findings of a research conducted on mice, the extract of Achillea sp. improved the wounds caused by Leishmania parasite in experimental animals [17].
TPs of Kabul city have used the infusion, decoction and powdered forms of A. cina for the treatment of diseases caused by Ascaris and beef tapeworm. The conducted studies stated that A. cina is one of the strongest anthelmintic plants, especially in children, which directly destroys roundworms, but has no effect on tapeworms. The essential oil and santonin are responsible for the anti-parasitic effect of this plant [18].
TPs have prescribed decoction of C. intybus root for patients after meals for the treatment of malaria. C. intybus is a plant traditionally used in some Asian, European and Middle Eastern countries to treat malaria [19]. The iridoids and lactocine are responsible for the antimalarial effect of C. intybus against P. falciparum [20].
A. absenthium has been used as an anti-parasitic medicine since the ancient Egyptians [21]. This plant is one of the most common plants used in the treatment of intestinal worms, and scientific research has shown that the alcoholic extract of A. absenthium is effective against T. cati and intestinal nematodes [22]. It is active against the malaria parasite, especially the drug-resistant type of P. falciparum [23].
F. assa-foetida is another herb used for the treatment of PDs, and the TPs of Kabul city have used its resin mixed with milk and yogurt to treat beef tapeworm and Ascaris. The results of the studies conducted in Iran, Nepal and China shows that F. assa-foetida is traditionally used for the treatment of intestinal parasites [24] and the sisquiterpenic coumarins present in the composition of the plant resin have an anti-leishmaniosis effect [25].
The TPs of Kabul city have used the fruits of different species of Apiaceae family including C. copticum, F. vulgare, C. sativum and A. graveolens, for the treatment of common PDs. The anti-parasitic effects of the mentioned MPs against different parasites have been studied. C. copticum has anthelmintic effects against Ascaris and Oxyur worms. The essential oil of F. vulgare fruit has lethal effects on malaria parasite vector larvae, and the oil of its leaves, flowers and root are effective against Culex pipiens mosquito larvae [26]. In some countries, the fruit of C. sativum is used to treat Ascariasis and hepatitis in humans. This plant is effective against nematode worms and H. nana, and this effect is due to the presence of the essential oil [27]. The flavonoid, phenolic, and essential oil of leaves, stems, and roots of A. graveolens are effective against giardiasis [28].
Eucalyptus sp. is another MP that is recommended by TPs in different districts of Kabul city for treatment of PDs. This plant shows considerable activity against Entameba histolytica and Trichomonas sp. [29]. The phenolic and non-phenolic extracts of Eucalyptus sp. leaves, which contain terpenic and phenolic phytoconstituents, are effective against Trichomonas sp. [30,31].
TPs have used the decoction and powdered form of G. glabra for the management of leishmaniosis and malaria. The anti-parasitic effects of G. glabra have been proven by scientific research. The results of these studies have made it clear that Glycyrrhizinic acid, glycyrrhetinic acid and licochalcone A present in the composition of G. glabra are responsible for the anti-parasitic effect of this plant, especially against Leshmania donovani infection [32].
T. indica is effective for the treatment of various PDs, especially malaria [33]. The tannins in this plant cause the destruction and death of intestinal parasites [34] and its fruit is used as an antipyretic in the treatment regimen of malaria [35].
Based on the findings of this research, the seeds of different MPs such as N. sativa, C. pepo, and L. usitatissimum are effective for the treatment of PDs. The powdered form of C. pepo seeds is used with honey to repel beef tapeworm. The seeds of C. pepo have anthelmintic properties in humans and other organisms [36,37] and are used to repel various worms, especially tapeworms. . In the same way, the seed of N. sativa is considered a good choice for the treatment of PDs. Studies conducted by Egyptian scientists have shown the anthelmintic effect of N. sativa against S. mansoni [38]. Researches have revealed that ethyl linoleate in L. usitatissimum protects mice deficient in vitamin E against the malaria parasite [39].
The dried peel of P. granatum is another medicine that the TPs of Kabul city have prescribed in the form of powder, infusion and decoction for the treatment of amoebiasis. The peels of P. granatum have an anthelmintic effect against S. mansoni [40]. The bark of stem, and root and fruit peels have been used since ancient times in various TMs for the treatment of intestinal PDs and diarrhea and even Dioscorides was prescribed its decoction to get rid of beef tapeworm. The methyl isopelletierine alkaloids present in the drug have selective effects against all kinds of tapeworms. The product called pelletierine tannate, which contains a set of alkaloids with tannic substances is used to get rid of tapeworms. In addition, due to the existence of a large amount of tannins, the peel of the fruit is used to treat diarrhea and dysentery.
Fumaria sp. has been used in TM for the treatment of various diseases since ancient times. Alkaloids, especially protopine, have anti-inflammatory effects and therefore its products are used in the treatment of skin manifestations such as scabies. Due to the presence of various alkaloids, this plant has anti-malarial and anti-nematode effects, and its ethanolic extract is traditionally used in Iran, Pakistan and India [41-43].
The bark of C. zeylanicum is another herbal drug used for the treatment of PDs. TPs has used the infusion of C. zeylanicum bark for the treatment of malaria. Studies have shown that the extract of C. zeylanicum bark has anti-plasmodial effect, which is done through inhibiting the enzyme enoyl-ACP-reductase [44]. In the same way, the research conducted on aqueous, hexane, methanolic and ethanolic extracts of C. zeylanicum bark have proven its activity against Leishmania and toxoplasma species [45,46]. Another report shows the strong effects of plant bark extract against Ascaris worm and nematode larvae in-vitro.
C. angustifolia is usually effective as an anthelmintic. It destroys parasites and expels worms from the intestines. The drug of this plant is used in combination with other anthelmintic plants (Z. officinale and F. vulgare) to treat roundworms. Phytoconstituents present in C. angustifolia, such as sennosides, are responsible for its anti-parasitic or anthelmintic effects [47].
TPs of Kabul city have used the gel of Aloe sp. leaves for the management of wounds due to leishmaniosis. The available evidence has shown that usage of the combination of Aloe sp. with C. longa extract for the treatment of leishmaniosis had good results [12]. The leaf extract of Aloe sp. has anti-protozoan activity against T. brucei parasite [48]. Similarly, the methanolic extract of the plant has shown remarkable activity against the parasite Trichomonas vaginalis [49]. Although the anti-malarial effect of Aloe sp. has not yet been proven, researches have shown that it is used in Tanzania and Kenya to treat malaria symptoms [50].
P. major, Lavadula sp., C. majus, B. officinalis and E. ribes are among the other MPs that TPs of Kabul city have recommended for the treatment of various PDs. According to the existing reports, the leaves of P. major are used as ant-parasitic and anthelmintic in Guatemala and Argentina [51]. Likewise, the essential oil of Lavadula sp. has anti-parasitic effect and inhibits the growth of T, vaginalis [52]. The sanguinarine alkaloid of C. majus is effective against Trichomonas sp. [53] and the plant latex is used to treat warts and roundworms [54]. B. officinalis is a strong repellent of body parasites, especially parasites of the digestive system, and shows good anthelmintic effects [55].
CONCLUSION
TPs in different areas of Kabul city have used a total of 29 MPs to treat common PDs. In general, most of the underground parts, herbs, fruits and leaves of the MPs are used in the form of decoction (mostly oral), infusion, extracts and powdered for the treatment purpose. TPs have set the dosage and the administration frequency of different preparations according to the severity of the disease, the overall health condition of the patient, the occurrence of any kind of side effects and allergic reactions etc. The effectiveness of using MPs to treat PDs by TPs in Kabul city, in most of the cases, matches the rational phytotherapy. As most of these plants have anti-parasitic effects and modern scientific research shows the mentioned activities. However, further studies are needed to ensure the identity, purity, quality and uniformity of MPs and their recommended dosage forms throughout the city.
RECOMMENDATIONS
- In order to ensure the safety and efficacy of recommended herbal remedies, it is suggested that more research and studies should be done for the approval of the identity, purity, quality and uniformity of MPs and their recommended dosage forms throughout the city.
- The results of scientific research conducted on MPs with anti-parasitic effects should be published nationwide. Publication of these studies not only increases the public awareness to use rational phytotherapy but also facilitate and improve the development of country’s TM.
REFERENCES
- Hassan HM. (2015). A Short History of the Use of Plants as Medicines from Ancient Times. Chimia (Aarau). 69(10):622-623.
- Ekor M. (2014). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 4:177.
- John DT, Petri WA. (2006). Markell and Voge's Medical Parasitology. Elsevier Health Sciences, United States.
- Hotez P, Herricks J. (2015). One Million Deaths by Parasites. PLOS. Available at: https://speakingofmedicine.plos.org/2015/01/16/one-million-deaths-parasites/
- Wink M. (2012). Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules. 17(11):12771-12791.
- Choi WH, Jiang MH, Chu JP. (2013). Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. J Appl Biomed. 11(1):15-26.
- Lin RJ, Chen CY, Lu CM, Ma YH, Chung LY, Wang JJ, Lee JD, Yen CM. (2014). Anthelmintic constituents from ginger (Zingiber officinale) against Hymenolepis nana. Acta Trop. 140:50-60.
- El-Nour MF, Fadladdin Y. (2021). Antischistosomal Activity of Zingiber officinale, Piper nigrum, and Coriandrum sativum Aqueous Plant Extracts on Hamster Infected with Schistosoma mansoni. Journal of Parasitology Research. 2021:6628787.
- El-Sayed NM, El-Saka MM. (2015). Anti-Parasitic Activity of Zingiber officinale (Ginger): A Brief Review. Aperito Journal of Bacteriology, Virology and Parasitology. 2(1).
- Kobo PI, Erin PJ, Suleiman MM, Aliyu H, Tauheed M, Muftau S. (2014). Antitrypanosomal effect of methanolic extract of Zingiber officinale (ginger) on Trypanosom a brucei brucei-infected Wistar mice. Veterinary World. 7(10):770-775.
- Almalki E, Al-Shaebi EM, Al-Quarishy S, El-Matbouli M, Abdel-Baki AS. (2017). In vitro effectiveness of Curcuma longa and Zingiber officinale extracts on Echinococcus protoscoleces. Saudi J Biol Sci. 24(1):90-94.
- Bahrami AM. (2011). Antileishmanial Effects of Traditional Herbal Extracts Against Cutaneous Leishmaniosis In Vivo. Adv Environ Biol. 5(10):3188-3195.
- Donipat P, Harasreeramulu S. (2015). In Vitro antimalarial activity of Rhizome extracts of Curcuma spicies. Journal of Survey in Fisheries Sciences. 6(4):B1141-B1146.
- Asadpour M, Namazi F, Razavi SM, Nazifi S. (2018). Comparative efficacy of curcumin and paromomycin against Cryptosporidium parvum infection in a BALB/c model. Vet Parasitol. 250:7-14.
- Santos AO, Santin AC, Yamaguchi MU, Cortez LE, Ueda-Nakamura T, Dias-Filho BP, et al. (2010). Antileishmanial activity of an essential oil from the leaves and flowers of Achillea millefolium. Ann Trop Med Parasitol. 104(6):475-483.
- Benedek B, Geisz N, Jäger W, Thalhammer T, Kopp B. (2006). Choleretic effects of yarrow (Achillea millefolium s.l.) in the isolated perfused rat liver. Phytomedicine. 13(9-10):702-706.
- Bahmani M, Saki K, Ezatpour B, Shahsavari S, Eftekhari Z, Jelodari M, et al. (2015). Leishmaniosis phytotherapy: Review of plants used in Iranian traditional medicine on leishmaniasis. Asian Pacific Journal of Tropical Biomedicine. 5(9):695-701.
- Higuera-Piedrahita RI, Dolores-Hernández M, de la-Cruz-Cruz HA, Andrade-Montemayor HM, Zamilpa A, López-Arellano R, et al. (2022). An Artemisia cina n-hexane extract reduces the Haemonchus contortus and Teladorsagia circumcincta fecal egg count in naturally infected periparturient goats. Trop Anim Health Prod. 54(2):95.
- Peña-Espinoza M, Valente AH, Thamsborg SM, Simonsen HT, Boas U, Enemark HL, et al. (2018). Antiparasitic activity of chicory (Cichorium intybus) and its natural bioactive compounds in livestock: a review. Parasit Vectors. 11(1):475.
- Bischoff TA, Kelly CJ, Karchesy Y, Laurantos M, Nguyen-Dinh P, Arefi AG. (2004). Antimalarial activity of lactucin and lactupichrin, sesquiterpene lactone isolated from C.I L. Antiparasitic activity of cichory and its natural bioactive compounds in livestock.
- Yousufi MR, Tabari MA, Hashjin GS, Kouhi MK. (2011). Antiparasitic efficacy of worm wood (Artemisia absinthium) alcoholic extract on syphacia obvolata. Iranian journal of veterinary medicine. 6(1):47-50.
- Yıldız K, Başalan M, Duru O, Gökpınar S. (2011). Antiparasitic efficiency of Artemisia absinthium on Toxocara cati in naturally infected cats. Turkiye Parazitol Derg. 35(1):0-4.
- Irshad S, Mannan A, Mirza B. (2011). Antimalarial activity of three Pakistani medicinal plants. Pakistan Journal of Pharmaceutical Sciences. 24(4):589-591.
- Babar W, Iqbal Z, Khan MN, Muhammad G. (2012). An inventory of the plants used for parasitic ailments of animals. Pakistan Vet J. 32:183-187.
- Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, Bassarello C, et al. (2007). Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry. 68(4):554-561.
- Sedaghat M, Dehkordi AS, Abai M, Khanavi M, Mohtarami F, Abadi YS, et al. (2011). Larvicidal Activity of Essential Oils of Apiaceae Plants against Malaria Vector, Anopheles stephensi. Iran J Arthropod Borne Dis. 5(2):51-59.
- Hosseinzadeh S, Ghalesefidi MJ, Azami M, Mohaghegh MA, Hejazi SH, Ghomashlooyan M. (2016). In vitro and in vivo anthelmintic activity of seed extract of Coriandrum sativum compared to Niclosamid against Hymenolepis nana infection. J Parasit Dis. 40(4):1307-1310.
- Sahib AS, Mohammed IH, Sloo SA. (2014). Antigiardial effect of Anethum graveolens aqueous extract in children. J Intercult Ethnopharmacol. 3(3):109-112.
- Iwiński H, Łyczko J, Różański H, Szumny A. (2022). Novel Formula of Antiprotozoal Mixtures. Antibiotics (Basel). 11(7):913.
- Mahdi NK, Gany ZH, Sharief M. (2006). Alternative drugs against Trichomonas vaginalis. East Mediterr Health J. 12(5):679-684.
- Hassani S, Asghari G, Yousefi H, Kazemian A, Rafieiean M, Darani HY. (2013). Effects of different extracts of Eucalyptus camaldulensis on Trichomonas vaginalis parasite in culture medium. Adv Biomed Res. 2:47.
- Bhattacharjee S, Bhattacharjee A, Majumder S, Majumdar SB, Majumdar S. (2012). Glycyrrhizic acid suppresses Cox-2-mediated anti-inflammatory responses during Leishmania donovani infection. J Antimicrob Chemother. 67(8):1905-1914.
- Nguta JM, Mbaria JM. (2013). Brine shrimp toxicity and antimalarial activity of some plants traditionally used in treatment of malaria in Msambweni district of Kenya. J Ethnopharmacol. 148(3):988-992.
- Das SS, Dey M, Ghosh AK. (2011). Determination of anthelmintic activity of the leaf and bark extract of tamarindus indica linn. Indian J Pharm Sci. 73(1):104-107.
- Ahmed AO, Ayoub SM. (2015). Chemical composition and antimalarial activity of extracts of Sudanese Tamarindus indica L. (Fabaceae). The Pharma Innovation Journal. 4(4):90-93.
- Marie-Magdeleine C, Hoste H, Mahieu M, Varo H, Archimede H. (2009). In vitro effects of Cucurbita moschata seed extracts on Haemonchus contortus. Vet Parasitol. 161(1-2):99-105.
- Alhawiti AO, Toulah FH, Wakid MH. (2019). Anthelmintic Potential of Cucurbita pepo Seeds on Hymenolepis nana. Acta Parasitol. 64(2):276-281.
- Ali M, Eldahab MA, Mansour HA, Nigm A. (2016). Schistosoma mansoni: Antiparasitic effects of orally administered Nigella sativa oil and/or Chroococcus turgidus extract. Acta Biol Hung. 67(3):247-260.
- Levander OA, Ager Jr AL, Morris VC, May RG. (1991). Protective effect of ground flaxseed or ethyl linolenate in a vitamin E-deficient diet against murine malaria1. Nutrition Research. 11(8):941-948.
- Abozeid KH, El-Badawy MF, Mahmoud S, Shohayeb MM. (2020). In vitro Effects of Punica granatum Ellagitannins on Adult Worms of Schistosoma mansoni. Res Rep Trop Med. 11:73-80.
- Gupta PC, Sharma N, Rao ChV. (2012). A review on ethnobotany, phytochemistry and pharmacology of Fumaria indica (Fumitory). Asian Pac J Trop Biomed. 2(8):665-669.
- Dwivedi A, Patel R, Jhade D, Sachan R, Argal A. (2009). Traditional Phytotherapy used in the Treatment of Malaria by Rural People of Bhopal, District of Madhya Pradesh, India. Ethnobotanical Leaflets. 13:475-479.
- Ghafari S, Esmaili S, Naghibi F, Mosaddegh M. (2013). Plants used to treat "taberebá" (malaria like fever) in Iranian traditional medicine. Int J Trad Herbal Med. 1:168-176.
- Nkanwen ERS, Awouafack MD, Bankeu JKK, Wabo HK, Mustafa SAA, Ali MS, et al. (2013). Constituents from the stem bark of Cinnamomum zeylanicum Welw. (Lauraceae) and their inhibitory activity toward Plasmodium falciparum enoyl-ACP reductase enzyme. Records of Natural Products. 7(4):296-301.
- Alanazi AD, Almohammed HI. (2022). Therapeutic Potential and Safety of the Cinnamomum zeylanicum Methanolic Extract Against Chronic Toxoplasma gondii Infection in Mice. Front Cell Infect Microbiol. 12:900046.
- Ghanbariasad A, Valizadeh A, Ghadimi SN, Fereidouni Z, Osanloo M. (2021). Nanoformulating Cinnamomum zeylanicum essential oil with an extreme effect on Leishmania tropica and Leishmania major. Journal of Drug Delivery Science and Technology. 63:102436.
- Kundu S, Roy S, Nandi S, Ukil B, Lyndem LM. (2016). Senna alexandrina Mill. induced ultrastructural changes on Hymenolepis diminuta. Journal of Parasitic Diseases. 41(1):147-154.
- Ivoke N. (2006). Preliminary studies on the efficacy of aloe vera (Aloe barbadensis ) extracts on experimental Trypanosoma brucei brucei infection of mice. Bio-Research. 3(1):21-25.
- Brandelli CL, Vieira Pde B, Macedo AJ, Tasca T. (2013). Remarkable anti-trichomonas vaginalis activity of plants traditionally used by the Mbyá-Guarani indigenous group in Brazil. Biomed Res Int. 2013:826370.
- Amir HM, Grace OM, Wabuyele E, Manoko ML. (2019). Ethnobotany of Aloe L. (Asphodelaceae) in Tanzania. South African Journal of Botany. 122:330-335.
- Samuelsen AB. (2000). The tradational uses, chemical constituent and biological activities of plantago major l. antihelmethic effect of plantago major L. in mice infected with aspiculuris tetraptera.
- Moon T, Wilkinson JM, Cavanagh HM. (2006). Antiparasitic effect of two lavandula essential oil against Giardia doudenalis trichomonas and hexamita parasit Ros. Parasitol Res. 99(6):722-728.
- Huang H, Yao J, Liu K, Yang W, Wang G, Shi C, et al. (2020). Sanguinarine has anthelmintic activity against the enteral and parenteral phases of trichinella infection in experimentally infected mice. Acta Tropica. 201:105226.
- Maji A, Banerji P. (2015). Chelidonium majus L. (Greater celandine) – A Review on its Phytochemical and Therapeutic Perspectives. International Journal of Herbal Medicine. 3(1):10-27.
- Choudhary GP. (2012). Anthelmintic Activity of Fruits of Embelia ribes Burm. International Journal of Pharmaceutical and Chemical Sciences. 1(4):1680-1691.
 Abstract
Abstract  PDF
PDF